
Research description
What happens when the body clock does not keep time?
It is now well known that long-term dysfunctional circadian rhythmicity has a detrimental effect on health and can contribute to severe diseases such as depression, diabetes, cardiovascular diseases and cancer.
I am interested in cellular mechanisms underlying circadian rhythmicity and how these can be altered in diseases and conditions that are characterized by sleep and rhythm dysfunctions.
Human physiology and behavior undergo daily (circadian = 24 hr) rhythms that are generated by an internal “body clock”, the hypothalamic suprachiasmatic nuclei (SCN). Rhythms may become disturbed by internal or external factors, for instance in “jet lag”, which occurs after rapid traveling across time zones. Disturbed rhythms are also common in certain psychiatric disorders, neurodegenerative diseases, African sleeping sickness and aging. Deciphering all components in the circadian machinery is a prerequisite for developing new therapeutic strategies to treat circadian rhythm disorders, and to bridge circadian research to the clinics.
Circa 24-h rhythmicity is generated by molecular feedback loops of rhythmically expressed genes and their protein products. The Nobel prize in medicine or physiology 2017 was awarded to Jeffrey Hall, Michael Rosbash and Michael Young for identifying this molecular feedback loop. Neuronal membrane activity, ion fluxes and cAMP also play a crucial role in circadian rhythm generation. I have used molecular imaging techniques to study neuronal rhythms from acute and organotypic SCN brain slices, and performed electrophysiological recordings in the acute SCN brain slice.
I am currently involved in projects with collaborators at Karolinska Institutet in which we study the cochlear clock and effects of auditory noise trauma (with Barbara Canlon) and genetic circadian components involved in Horton's disease (with Andrea Carmine Belin). In clinical collaboration projects we also study rhythmic gene expression in fibroblasts from patients with schizophrenia and bipolar disorder (with Jerker Hetta, Björn Owe-Larsson and Anne-Sofie Johansson).
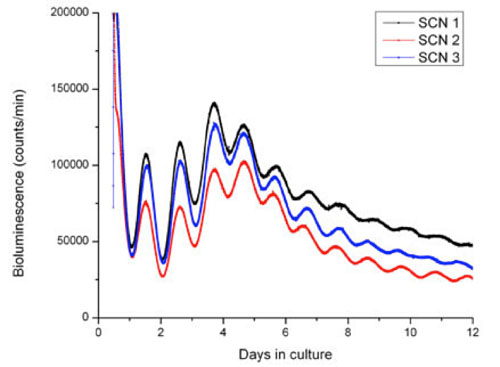
Studying the molecular clock ticking in real time
The circadian pacemaker is located in the “suprachiasmatic nucleus” (SCN) in hypothalamus. The molecular circadian clock activity in the SCN is in the figure visualized using dynamic luciferase reporter technology. The figure shows raw data of luciferase activity monitored as bioluminescence (photon emission) in SCN tissues, which corresponds to the daily expression of the PERIOD 2 protein, one of the key players in the molecular clock.
Three organotypic tissues (represented by black, red and blue) containing the SCN from transgenic mice held in a 12 hr light:12 hr dark cycle were cultured for 12 days. The PERIOD 2 protein expression is typically high at the onset of subjective darkness and low at onset of subjective day.



